Drug to address alopecia authorized by Food and drug administration
NEWYou can now pay attention to G3 Box News articles!
The Food items and Drug Administration (Food and drug administration) announced Tuesday that it permitted Olumiant oral tablets for adult patients who are afflicted by extreme alopecia areata.
The move by the company marks the 1st Fda acceptance of a systemic remedy for the dysfunction.
“Obtain to safe and sound and productive remedy solutions is very important for the important quantity of Us residents affected by serious alopecia,” Dr. Kendall Marcus, the director of the Division of Dermatology and Dentistry in the FDA’s Middle for Drug Analysis and Study, reported in a assertion. “Today’s acceptance will enable satisfy a important unmet need to have for individuals with severe alopecia areata.”
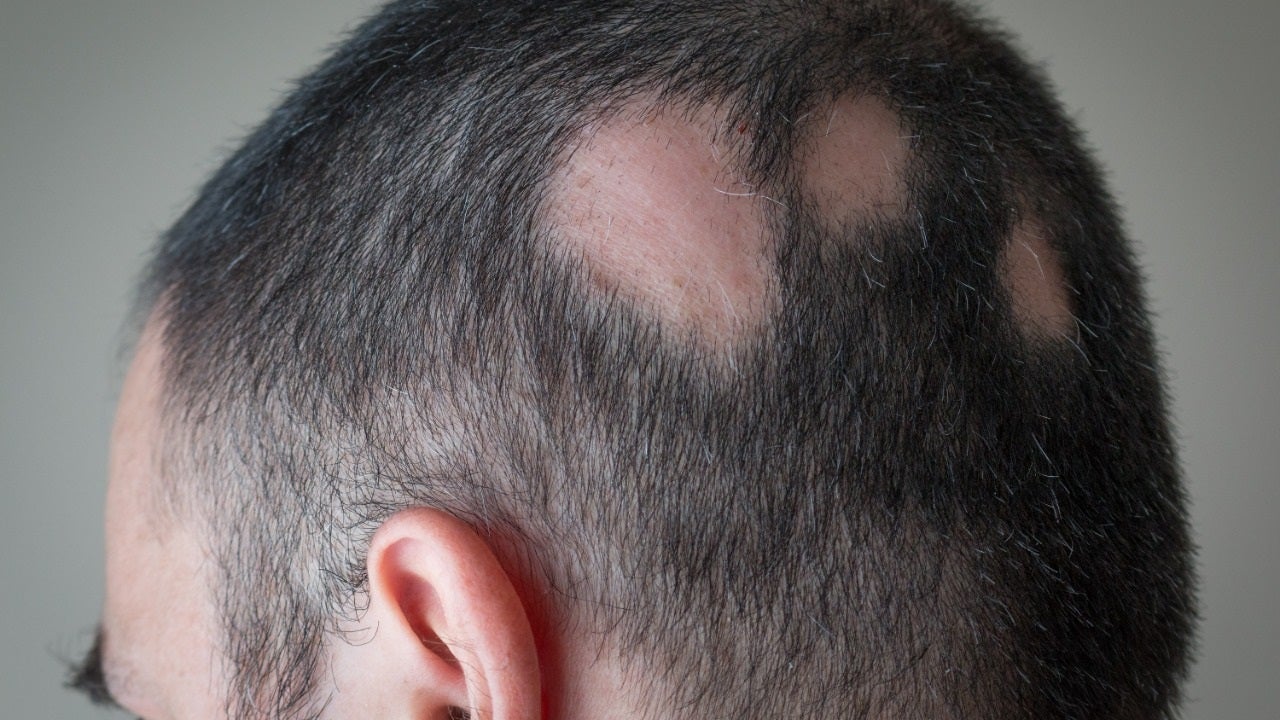
Generally referred to as just alopecia, alopecia areata is an autoimmune ailment that develops when the physique assaults its personal hair follicles, which can induce hair decline anyplace on the human body.
US MONKEYPOX Bacterial infections CLIMB TO 65
In accordance to the American Academy of Dermatology Association, it can begin at any age, although most individuals produce it through childhood or their teenage yrs.
There are various styles of alopecia areata, which include alopecia totalis and alopecia universalis.
The condition, which impacts far more than 300,000 Us residents each yr, frequently seems as patchy baldness.
The acceptance for the drug was granted to Eli Lilly.
The tablets are a Janus kinase (JAK) inhibitor, which blocks the activity of a person or much more of a precise loved ones of enzymes, interfering with the pathway that sales opportunities to swelling.
Its efficacy and basic safety ended up tested in two randomized, double-blind and placebo-controlled trials.
WHAT IS RAMSAY HUNT SYNDROME? JUSTIN BIEBER REVEALS Prognosis
Individuals experienced at minimum 50% scalp hair decline, as measured by the severity of alopecia tool, for a lot more than 50 % a 12 months.
The people either been given a placebo, 2 milligrams of Olumiant, or 4 milligrams every working day.
The Food and drug administration explained that the principal measurement of efficacy for equally trials was the proportion of patients who had attained at minimum 80% scalp hair protection at 7 days 36.
“In Demo AA-1, 22% of the 184 people who received 2 milligrams of Olumiant and 35% of the 281 clients who acquired 4 milligrams of Olumiant realized enough scalp hair protection, as opposed to 5% of the 189 people who obtained a placebo. In Demo AA-2, 17% of the 156 people who received 2 milligrams of Olumiant and 32% of the 234 individuals who received 4 milligrams of Olumiant realized enough scalp hair coverage, in contrast to 3% of the 156 clients who been given a placebo,” the Food and drug administration mentioned.
The most common facet effects linked with Olumiant consist of upper respiratory tract bacterial infections, headache, acne breakouts, significant cholesterol, fatigue, nausea and pounds boost.
Click Listed here TO GET THE G3 Box News Application
It was in the beginning permitted in 2018 as a treatment method for specific grownup individuals with reasonably to seriously active rheumatoid arthritis and is also accepted for the cure of COVID-19 in sure hospitalized grownups.
It is not advised for use in mixture with other JAK inhibitors, biologic immunomodulators, cyclosporine or other potent immunosuppressants.